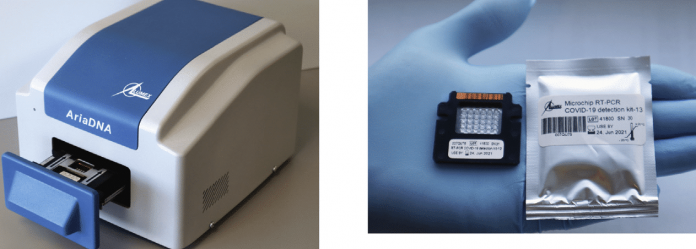
Simon Fraser University researchers have validated a faster, RT-PCR-based diagnostic assay kit useful for widespread COVID-19 testing. The test kit is approved by the Centers for Disease Control and Prevention (CDC), and the study results have been published in The Journal of Molecular Diagnostics (Cojocaru et al. 2021).
“This research offers a cheaper, faster alternative to the most reliable and sensitive test currently used worldwide, without sacrificing sensitivity and reproducibility,” says molecular biology and biochemistry professor Peter Unrau, who led the team evaluating the COVID-19 test kit.
The development of rapid, sensitive, and cost-effective diagnostics for COVID-19 testing was essential for providing medical care to people infected with the virus and the containment of the virus from the spread.
But, most of the current available RT-PCR assays require expensive reagents in high volume. They have significant technical labor for preparing complex reagent mixtures, resulting in increased costs with possible human error.
This new COVID-19 test kit requires ten times fewer reagents than the tube-based RT-PCR tests and can provide accurate results in 30 minutes. The machine could be deployed in remote locations, clinics, and airports due to its ease of use and portability.
To validate, the researchers sent the kit to a clinical team at St. Paul’s Hospital in Vancouver, British Columbia, Canada. There the team used supplied reference samples and 21 clinical samples from patients in the hospital.
The validation team found that the newly developed microchip PCR COVID-19 test kit results were comparable with hospital testing results.
The team stated that the real-time microchip PCR provides a significantly lower resource alternative to the CDC–approved real-time RT-PCR assays with comparable sensitivity, showing 100% positive and negative predictive agreement of clinical samples.
The PCR system consists of a disposable microchip with 30 microreactors (6 columns × 5 rows) where each well can accommodate a miniature TaqMan chemistry-based reaction of 1.2 μL.
The protocol uses a single-plex assay based on one-step RT-qPCR reactions targeting the SARS-CoV-2–specific N gene.
Since the outbreak of the pandemic (COVID-19) in Wuhan, China, the virus SARS-CoV-2 caused the disease, spread rapidly. As of May 22, 2021, while writing this report, over 166 million cases and 3,458,945 deaths (Worldometers 2021) have been reported worldwide, resulting in an enormous economic impact.
Because the numbers of infections and transmissions continue to increase, there is an urgent need for rapid and inexpensive diagnostic tests. The low-energy assay system (100 W) is available internationally. It’s cost-effective, compact, and lightweight.
Therefore, the system can be installed in a clinical diagnostic laboratory. The machine can also be used in remote locations such as airports, clinics, maintain similar comparable test results as observed with traditional RT-qPCR.
The detection kit, developed by Lumex Instruments Canada and validated by Unrau’s team, demonstrates a promising versatile technology. It can be easily configured and mobilized to detect infections of current and future viruses, overcoming the current crisis and ensuring a rapid response in the future, the report stated.