COVID-19 infections elevated the levels of interleukin-6 (IL-6) in severe and mortal COVID-19 cases. However, this study did not find any association between the levels of IL-6 with the severity and mortality of the patients as predicted in the earlier analysis.
Interleukin 6, a cytokine, an important biomarker of inflammation, contributes to host defense against infection. However, excessive production of cytokine, including IL-6, can lead to severe acute inflammatory response, known as a ‘cytokine storm,’ which increases the risk of multiorgan failures and death. The biomarker IL-6 has been shown to be elevated in many severe COVID-19 patients. Still, limited studies validated if there is any positive and significant correlation between the increase of IL-6 with COVID-19 severity and mortality.
Methods
This study conducted a relevant literature search in PubMed and Google Scholar using the keywords—interleukin-6, cytokine storm, severity, and mortality—combined with Covid-19, SARS-CoV-2, or coronavirus on August 10, 2020. The study retrieved over a hundred articles. After reviewing the title and abstract, the literature was shortlisted.
Finally, the study selected 35 articles for full-text review. The studies that divided enrolled patients into two arms, severe vs. non-severe or survivor vs. mortal, were included for meta-analysis. Severe is defined as the patients admitted to ICU, requiring mechanical ventilation, diagnosed with low oxygen saturation level (≤ 93%), or declared refractory, deteriorated, or severe in the records.
Mean serum IL-6 levels were compared, and mean difference (MD) was calculated with a 95% confidence level (CI), using the Inverse-Variance method and Random Effects model. For meta-regression, scatter plots were generated, estimated coefficient (Q) at 95% CI with P<.05 considering statistically significant. The study also attempted meta-regression to evaluate the impact of the moderator variable, IL-6, on the effect sizes, severe or death, in this analysis.
Results
A total of 16 studies (Cai et al., 2020, Chen et al., 2020, Huang et al., 2020, Li et al., 2020, Luo et al., 2020, Lv et al., 2020, Mo et al., 2020, Ruan et al., 2020, Tu et al., 2020, Wang et al., 2020, Wang et al., 2020, Wang et al., 2020, Wei et al., 2020, Yan et al., 2020, Zhang et al., 2020, Zhou et al., 2020) involving 4384 patients enrolled in this study. All studies were conducted in China.
Of the total studies, nine involving 1846 patients were enrolled in non-severe vs. severe cohorts (Cai et al., 2020, Chen et al., 2020, Huang et al., 2020, Li et al., 2020, Lv et al., 2020, Mo et al., 2020, Wang et al., 2020, Wei et al., 2020, Zhang et al., 2020); on the other hand, eight studies involving 2538 patients participated in the death vs. survivor cohorts (Luo et al., 2020, Ruan et al., 2020, Tu et al., 2020, Wang et al., 2020, Wang et al., 2020, Yan et al., 2020, Zhou et al., 2020).
Mean IL-6 Level
Data analysis revealed that overall pooled mean IL-6 level in poor outcome (severe and death) was higher (50.360 pg/mL, CI: 41.658 to 59.061, P < 0.001) compared with overall good outcome (non-severe and survivors), (MD 38.51, 95% CI 30.15 to 46.87; participants = 4384; studies = 17; I2 = 100%), Figure 1.

Subgroup analysis showed that patient who were severe had higher pooled mean IL-6 values (42.347pg/mL, CI: 33.333 to 51.361 P< 0.001) than the non-severe patients (MD 29.32, 95% CI 22.05 to 36.59; participants = 1846; studies = 9; I2 = 98%).
Mortal patients had even higher IL-6 (58.481 37.961 79.002 10.470 < 0.001) level compared with the survivors, (MD 46.92, 95% CI 27.28 to 66.56; participants = 2538; studies = 8; I2 = 100%).
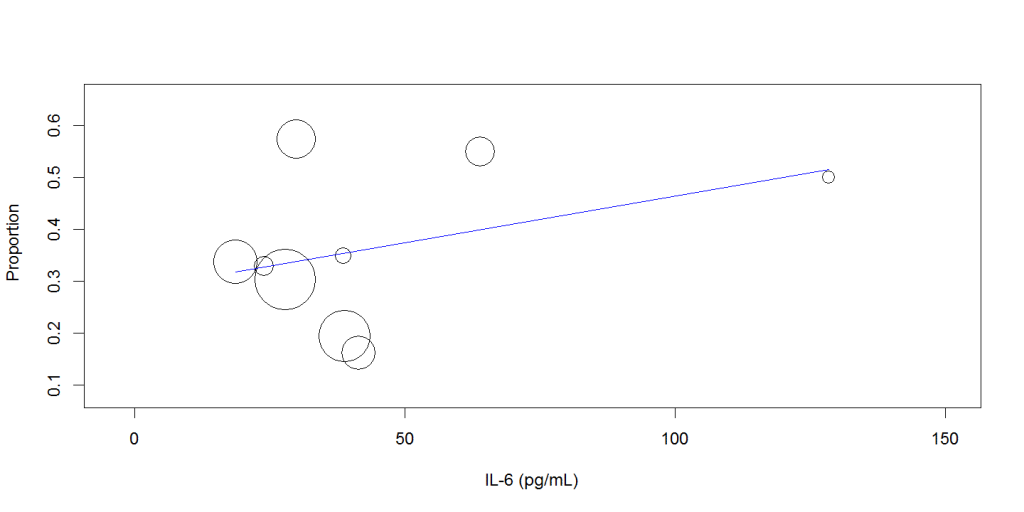
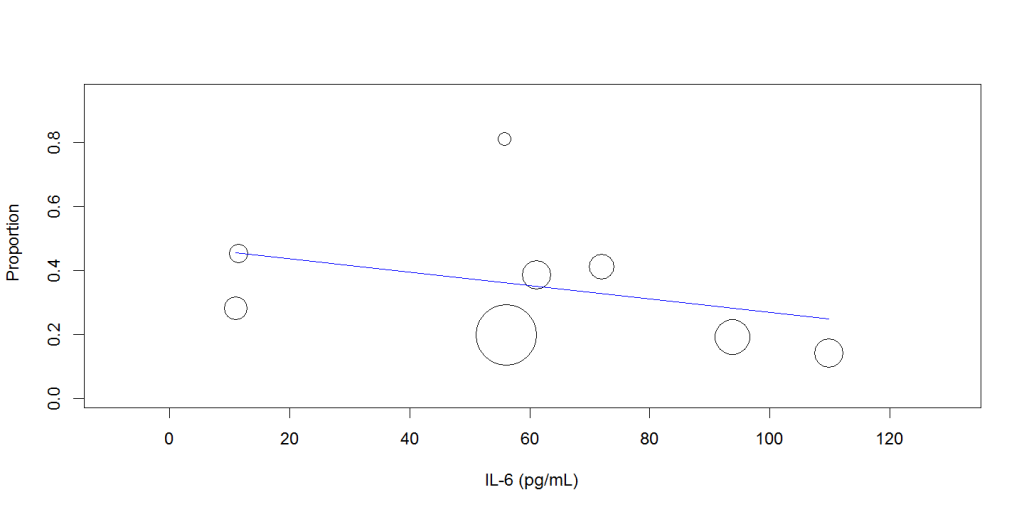
Random-effects meta-regression analysis showed that increasing mean IL-6 level was not associated with the disease severity (Q: 0.002, 95%CI: -0.001 to 0.005, P= 0.256), Figure 2, or mortality (Q: -0.002, 95% CI: -0.006 to 0.002, P=0.289), Figure 3.
Discussion
Compared with two other meta-analyses that included 1,426 (Aziz et al., 2020) and 3,400 patients (Zhu et al., 2020), this meta-analysis included 4384 patients. In agreement with the previous analysis, this study also found significantly higher mean IL-6 values in dead/severe patients than in non-severe/survivor patients. D
Therefore, this result agrees with the idea that severe patients had comparatively higher values of IL-6 level. However, this analysis did not see any significant correlation between the increasing mean of IL-6 level and an increased proportion of disease severity or mortality, in contrast to the first study (Aziz et al., 2020), which found a positive correlation with mortality and increased serum IL-6 level.
Several ways this study can explain the disparity between the two studies. First, this study has included more studies involving a large number of patients. Secondly, this study did not exclude any study that may be considered biased. Even though the study did not find any significant correlation, the observation may be light shading. This observation does support the idea that the pathogenesis of COVID-19 patients is not unique, and many other factors such as age, sex, existing comorbidity, medications, and poverty substantially modify disease outcomes in COVID-19 patients.
In summary, elevated IL-6 levels may be observed in severe COVID-19 patients; however, this is not the only parameter clinicians should use to recognize critical patients.
The author declares no conflict of interest.
Author: Molay Roy, PhD; Orchid ID: 0000-0002-8236-7265



















